Disabled macroautophagy
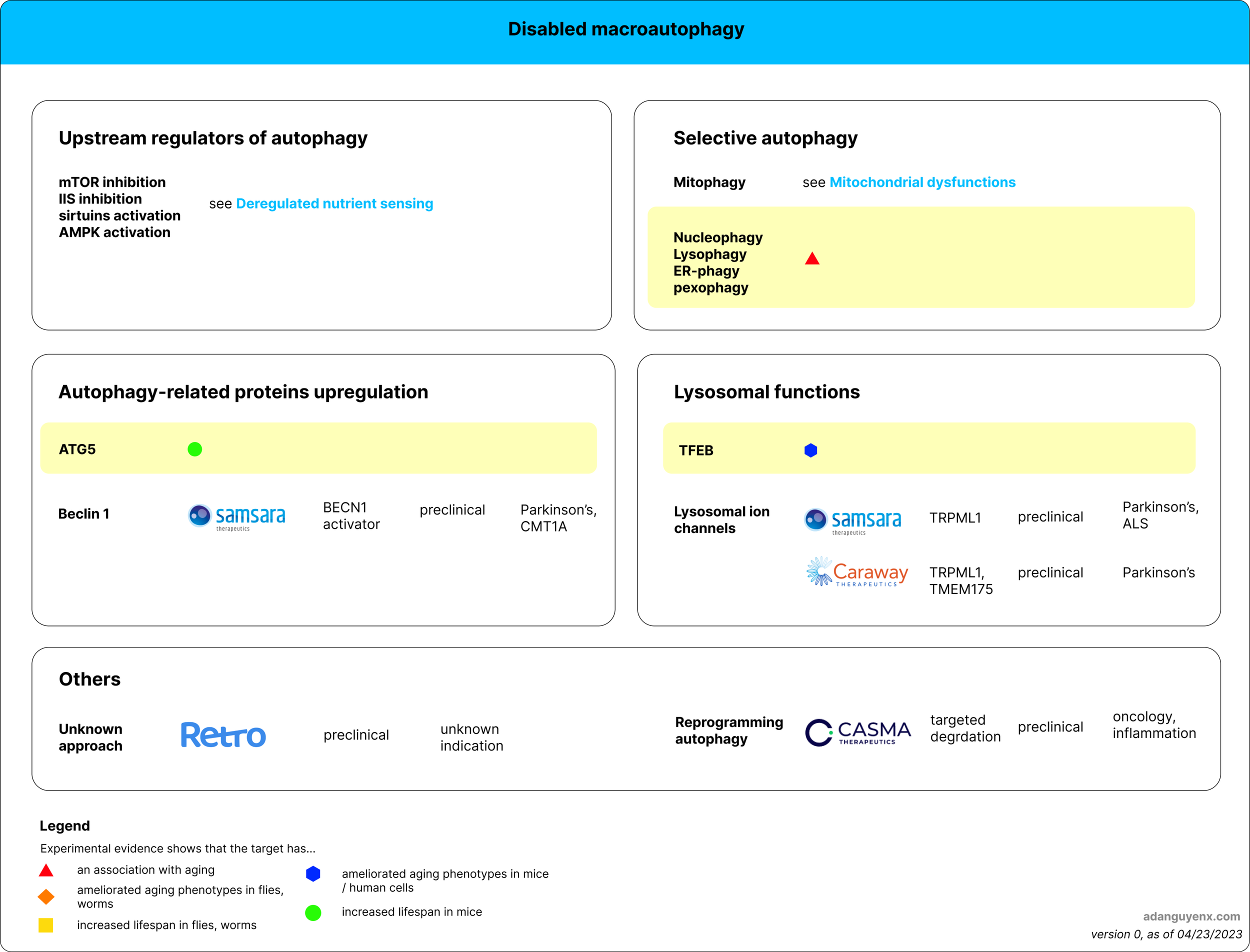
Figure 1: Therapeutic approaches for disabled macroautophagy. Areas with yellow highlight indicate target(s) not explored by existing companies. Company logo is followed by description of their approach, stage of development, and disease(s) they target.
Autophagy refers to the various pathways used by cells to transport cytoplasmic substances to lysosomes for degradation. These pathways include macroautophagy, chaperone-mediated autophagy (CMA), and microautophagy. While CMA has been covered in the “Cell aggregates” section, little is known about the relevance of microautophagy in mammalian organismal aging, and this pathway remains poorly studied. Therefore, this discussion will only focus on macroautophagy, also commonly called simply as autophagy.
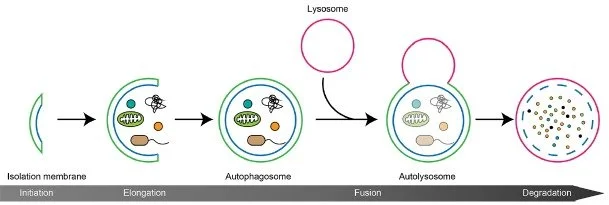
Figure 2. Autophagy process (source: Nakamura and Yoshimori 2018)
The process of autophagy can be broken down into the following steps:
Initiation: This step involves the activation of a complex of proteins called the ULK1 complex, which is regulated by several signaling pathways, including mTOR, AMPK, and IIS
Autophagosome formation and maturation: The process begins with the creation of an isolation membrane, or phagophore, which envelops the cytoplasmic component that needs to be broken down. This membrane then extends and seals itself, forming a double-membraned structure known as the autophagosome. As the autophagosome matures, it acquires various proteins and lipids essential for its eventual fusion with a lysosome.
Fusion and degradation: In the final step, the autophagosome merges with a lysosome to create an autolysosome. Lysosomal enzymes then break down the contents of the autophagosome, and the resulting degradation products are released back into the cytoplasm for reuse by the cell.
Several animal models have shown a decline in autophagy with aging, while upregulating autophagy can increase lifespan. By targeting the components involved in each step above, autophagy can be stimulated, resulting in improvements in healthspan.
upstream regulators of autophagy
Autophagy is induced by various cellular stresses, such as nutrient deprivation, hypoxia, and accumulation of damaged proteins or organelles, so its regulation is tightly linked to several cellular signaling pathways, such as mTOR, IIS, and AMPK. One approach to enhancing autophagy is to target these pathways, which are discussed in the "Deregulated nutrient sensing" section.
2. autophagy-related (ATG) proteins
2.1. Beclin
Beclin, the mammalian homolog of yeast protein ATG6, is a protein that plays a crucial role in autophagosome formation and lysosomal fusion. Activation of Beclin through disrupting the beclin 1–BCL2 complex increases autophagy, extends lifespan, and reduces age-related renal and cardiac changes and spontaneous malignancies in mice (Fernández et al. 2018). Samsara Therapeutics is currently developing Beclin activators for Parkinson’s disease.
2.2. ATG5
ATG5 is another key protein involved in autophagosome formation. Overexpression of ATG5 activates autophagy, extends lifespan, and confers a lean phenotype with increased insulin sensitivity and resistance to oxidative stress in mice (Pyo et al. 2013).
Unlike Beclin, there is a lack of well-defined methods to activate ATG5, and no companies are targeting it for autophagy. This presents an unexplored opportunity to target ATG5 for aging.
3. lysosomal proteins
The fusion of autophagosomes with lysosomes, followed by degradation, is an essential final step in the autophagy process. Enhancing lysosomal functions can lead to improvements in autophagy as well. Furthermore, it has been demonstrated that lysosomal proteins play a multifaceted role in autophagy, such as creating a positive feedback loop to increase autophagic flux (Huang et al. 2020). This highlights the importance of lysosomal proteins in autophagy beyond just the final step.
3.1. Lysosomal ion channels
TRPML1 and TMEM175 are both proteins found on lysosomal membranes that function as ion channels. They play a role in maintaining proper lysosomal function, and their dysfunction has been implicated in decreased autophagy and age-related diseases such as Parkinson’s (Tedeschi et al. 2019, Jinn et al. 2017).
Samsara Therapeutics and Caraway Therapeutics are both developing activators for one or both of these ion channels for Parkinson’s disease.
3.2. TFEB
TFEB is a transcription factor responsible for lysosome biogenesis. Overexpression of HLH-30, a C. elegans homolog of TFEB, extends lifespan in an autophagy-dependent manner in worms (Lapierre et al. 2013). Small molecules that activate TFEB also ameliorate metabolic syndrome in mice and extend lifespan in worms (Wang et al. 2017).
While there are many ways to design TFEB agonists (Chen et al. 2021), it’s unclear if there are any companies that directly activate TFEB for autophagy. TFEB therefore presents a potential underexplored target for aging.
4. Selective autophagy
Selective autophagy is a specific type of autophagy that involves the targeted degradation of specific cellular components. Selective and non-selective autophagy share several of the steps described above. One exception is that during autophagosome formation, selective autophagy recognizes specific components through specialized proteins that can bind to specific cargo, while non-selective autophagy forms autophagosomes around portions of the cytoplasm without specific selection. Targeting the components involved with these specialized receptor proteins is another strategy to upregulate selective autophagy.
4.1. Mitophagy
One of the most studied forms of selective autophagy is mitophagy, which involves the targeted degradation of mitochondria. PINK1 plays a key role in mitophagy by accumulating on the mitochondrial membranes in response to damage and recruiting Parkin. This results in the ubiquitination of outer mitochondrial membrane proteins, which serves as a signal for the autophagy machinery to recognize and engulf the damaged mitochondria. Parkin overexpression increases lifespan in flies (Rana et al. 2013), thus increasing PINK1/Parkin level is a strategy to increase mitophagy and extend healthspan. Both Vincere Biosciences and Mitokinin are pursuing this approach for Parkinson’s disease, with Vincere focusing on Parkin activation and Mitokinin on PINK1 activation.
4.2. Other selective autophagy
Other types of selective autophagy, including nucleophagy (nucleus), aggrephagy (proteins), lysophagy (lysosomes), pexophagy (peroxisome), ER-phagy have been associated with aging (Papandreou and Tavernarakis 2021, Papandreou et al. 2022), but there is no direct evidence that upregulation of these would extend lifespan in model organisms yet.
As of now, there are no companies actively pursuing these additional forms of selective autophagy, which presents an opportunity to explore their potential as therapeutic targets.
5. Other approaches
Casma Therapeutics has an interesting approach, in which they are creating a platform to generate autophagosomes around disease targets – inventing their own kind of selective autophagy. Retro Biosciences is another company working on autophagy, but it is unknown what their approach is.
Autophagy is a highly promising process with multiple targets, and several companies are focusing on different aspects of it. However, it's important to note that excessive autophagy can have harmful consequences. In fact, inhibiting autophagy in the later stages of life has been shown to increase the lifespan of worms (Wilhelm et al. 2017). Furthermore, pinpointing particular pathways and substrates of autophagy that possess anti-aging properties may pose potential difficulties, as autophagy is a complex process.